Our current projects:
The mechanistic source of p53 specificity and it relationship to the sequence-dependent structural code of DNA readout by p53.
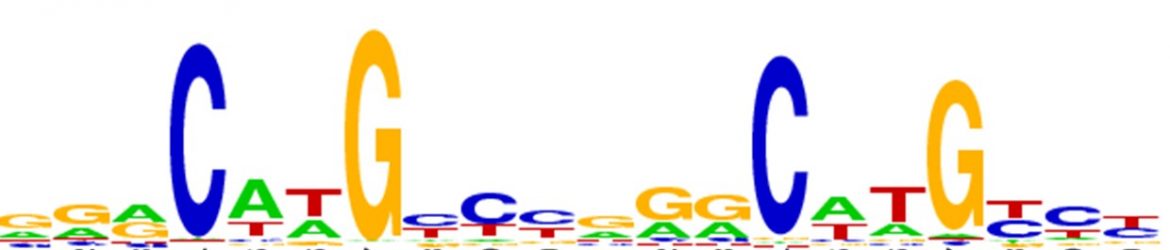
Based on our previous study (Jordan et al. 2012) we will assess the hypothesis that at least at basal p53 levelsת p53 specificity is encoded in the kinetic stability of the p53-DNA (p53-response elements) complexes, rather than in the binding affinity of these complexes. To answer these questions, we conduct high-throughput selection studies using SELEX-seq as a function of thermodynamic versus kinetic selection criteria at several different limiting protein concentrations.
Is the binding specificity related to the structure and mechanical properties of p53 response elements
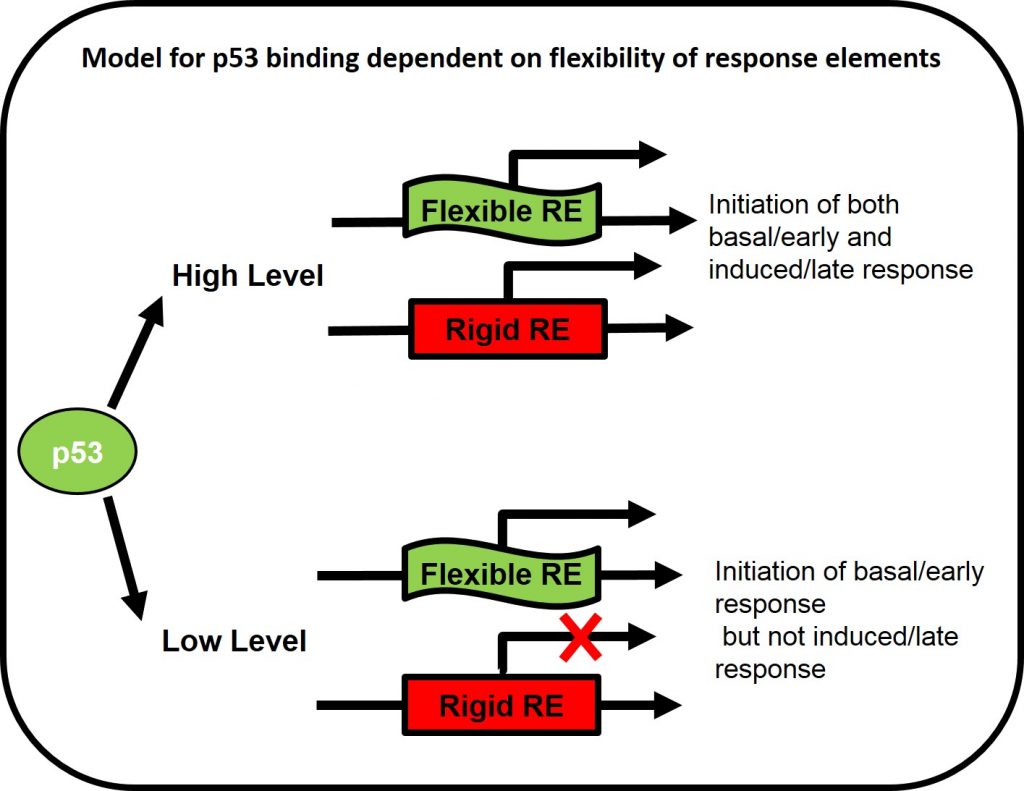
Upon stress, p53 level increases, it binds to its response element’s (RE’s) and initiate transcription. p53-dependent genes can be divided into two main groups, basal/early response and induced/late response. The result of initiating one of the response pathways in the right time and circumstances is critical for cell life. Taking together the observation that p53 level is highly dynamic and that the destructive consequences of committing wrongly to an irreversible fate, an important question is – how p53 bind to the correct RE in the right time at such a complex system? and more specifically does p53 binding and transctivation of adjacent genes is dependent on DNA flexibility of the response elements? To answer this question we measure the transactivation of p53 RE from different functional groups in basal and induced p53 levels in vivo using reporter gene assays in mammalian cells (H1299 p53-null cells).
The role played by the structural properties of spacer sequences of p53 response elements
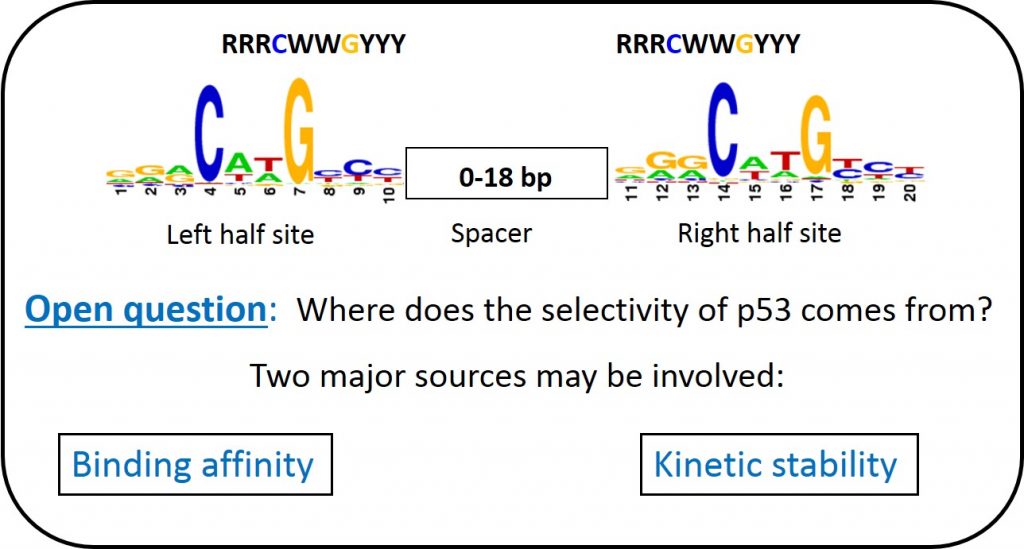
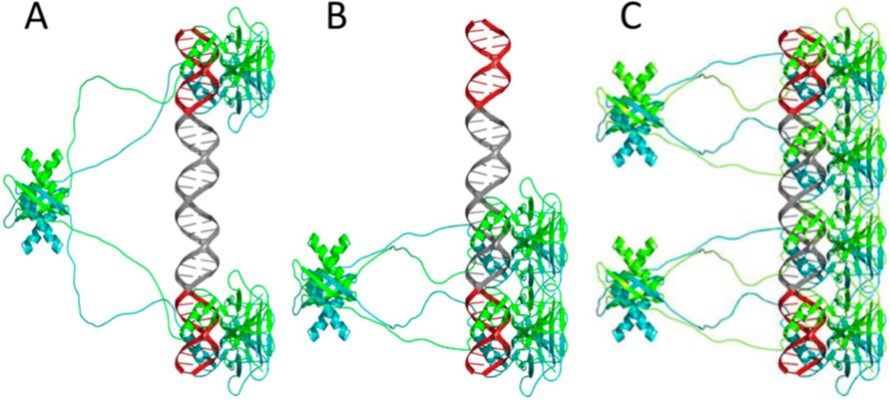
50% of all known p53 REs have spacer sequence (up to 18-bp long) between the two decameric half-sites. The binding of p53 is highly cooperative and p53 tetramerization occurs on DNA (figure ?). An open question was how p53 binds cooperatively to REs having spacers. We recently showed that p53 binds to response elements containing long spacers in two different modes: fully-specific and hemi-specific (Vyas et al. 2017). In the hemi-specific mode, only one p53 dimer is specifically bound to a DNA half-site, whereas the other dimer is bound to the spacer DNA. An unexpected result is that these two binding modes have comparable binding affinities, and moreover, the hemi-specific tetramer complex competes better than the full-specific tetramer against bulk genomic DNA (Vyas et al. 2017). Thus, this new binding mode is highly likely to play a major role in p53 functionality.
Many p53 binding sites were shown to contain an isolated p53 half-site. The hemi-specific binding mode can be used to create functional tetramer complexes from such sites. Moreover, many p53 sites contain a cluster of a canonical full-site together with an adjacent secondary half-site (or an additional full-site). The hemi-specific binding mode could bridge between the main and the secondary (or additional) sites. We are investigating the role played by the structural properties of spacer sequences of p53 REs, by conducting binding and transactivation assays as a function of the structural properties of the spacer sequences.
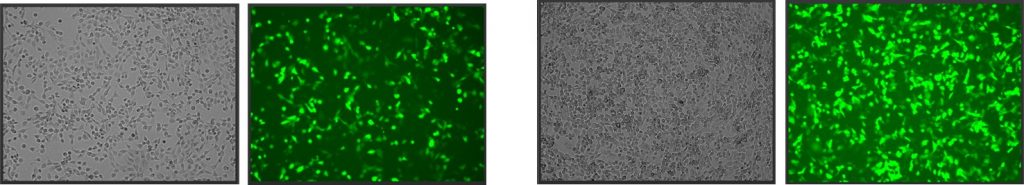
Mechanism of gain of function GOF in cancer
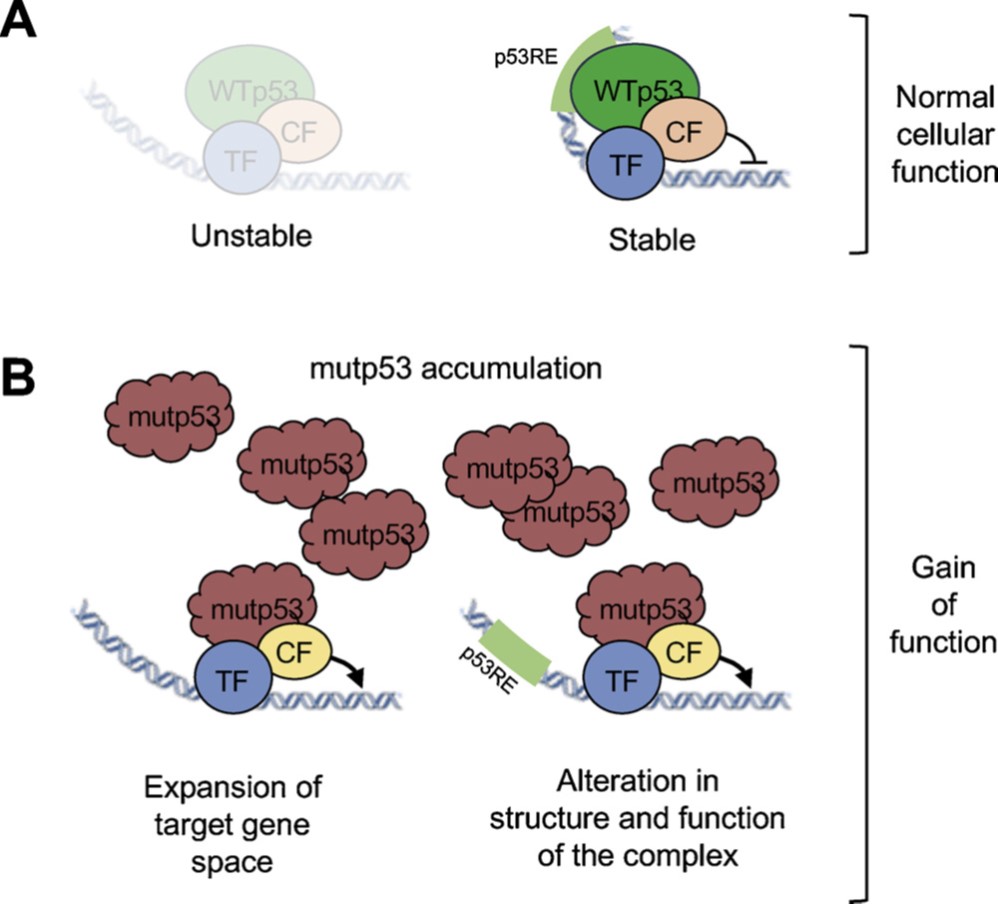
~50% of all tumors carry mutations within the TP53 gene, disrupting p53’s tumor suppressor activity (loss-of-function mutations, LOF) and may also switch the mutated p53 (mutp53) protein into an actively tumor-promoting oncogene (gain-of-function mutations, GOF). There appears to be LOF and GOF gradients, with different extent of LOF or GOF among various mutants. We recently suggested a new mechanism of mutp53 GOF, through cooperation with other transcription factors (TFs) (Stiewe &Haran, 2018) Figure 6. In wild type p53/transcription factor (wtp53/TF) complex both proteins contact the DNA on their respective specific sites, but the DNA contacts to p53 are lost in mutp53. We hypothesize that in this case mutp53 can stay bound to the auxiliary TF, which can result in a large expansion of target gene space, since the auxiliary TFs are mostly general transcription factors. Such a mechanism may hold true mostly for mutp53 proteins that are completely devoid of DNA binding capabilities. Mutp53 with partial DNA binding ability, may still display GOF activity, but their mechanism of action will most likely be a different one. We are currently validating this model.